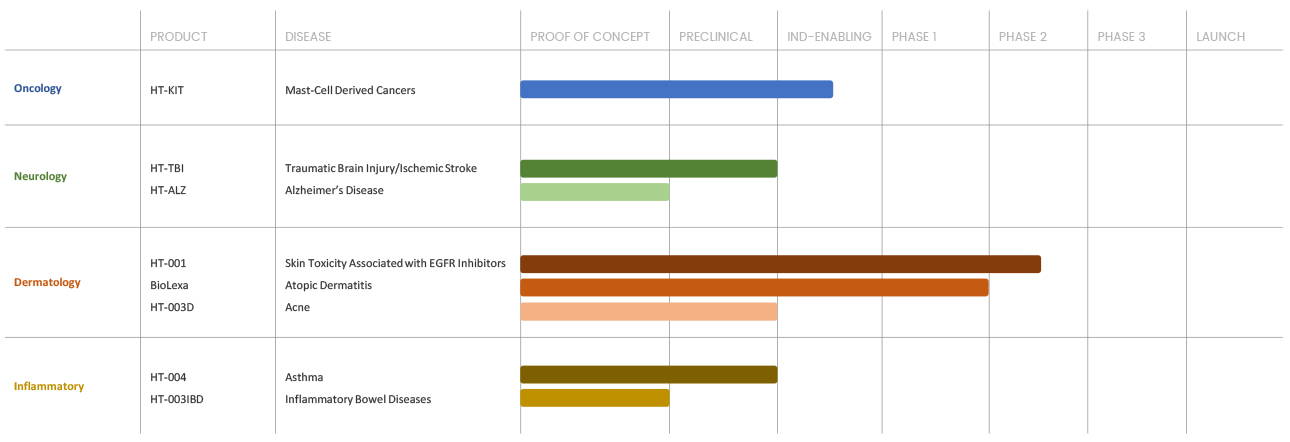
Our Progress
We are committed to the patient experience by following the science to develop impactful and versatile treatment solutions for everyone. At HOTH, we stand by our mission to innovate today for a better tomorrow.
Learn more about our therapeutic areas, Early Access program, as well as ongoing and future clinical trial opportunities.
If you have questions regarding our clinical trials, please contact us here.
HOTH is a catalyst in early-stage pharmaceutical research and development, elevating drugs from the bench to pre-clinical and clinical testing. Utilizing a patient-centric approach, we collaborate and partner with a team of scientists, clinicians, and key opinion leaders to seek out and investigate medications that hold immense potential to create breakthroughs and diversify treatment options.
Our current assets are focused in treating a broad range of diseases, including cancer, acne, inflammatory bowel disease, asthma, atopic dermatitis, skin toxicities associated with cancer therapy, TBI/ Stroke and Alzheimer’s disease.